100% empty vial inspection for vaccine production
The worldwide production capacities for the manufacture of vaccines are currently running at full speed. Nevertheless the Covid-19 vaccine, which is required in large quantities, remains in short supply so that every single dose counts. HEUFT is equipping one of the new production lines with an empty vial inspection so that there are no compromises with regard to packaging safety.
The new development extends far beyond the regulations for parenteral products which require the filled vials to be subjected to 100% integrity testing. This is because it examines this primary packaging at an earlier stage when it is still empty.
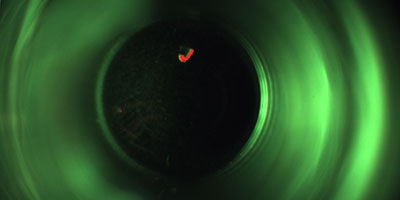
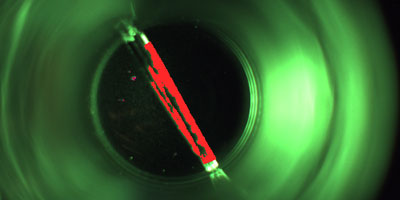
The aim is to detect faulty vials which are contaminated with minute glass splinters caused by possible glass breakages in the hot sterilisation tunnel even before the actual filling process. This ensures that the valuable vaccine does not find its way into the packaging in question in the first place.
Fullscreen
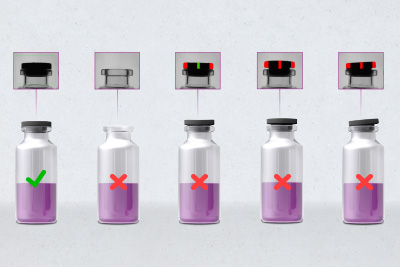
100% integrity testing for each individual empty vial
Therefore the in-house developed smart sensor camera with special lighting and integrated image processing from HEUFT will be installed directly above the infeed starwheel of the filling machine of a new production line for a Covid-19 vaccine which is currently being built in Germany. It will ensure that each individual vial is subjected to a 100% integrity test along the line where a contract manufacturer will produce and pack 12,000 doses per hour under aseptic conditions from the middle of 2021.
The inspection, which is in a top-down arrangement, is also capable of detecting other contamination, cracks and further faults on the base of the vials as well as minute glass splinters. The position of the containers in question is tracked precisely so that they cannot be filled with the liquid inoculant under any circumstances.
Continuous audit trail and complete documentation
The innovative detection unit for an empty vial inspection is connected to a compact HEUFT SPECTRUM II control panel with a hygiene-optimised stainless steel casing. The integrated audiovisual HEUFT NaVi user guidance meets the FDA 21 CFR Part 11 requirements with personalised access rights and a continuous audit trail log. A current HEUFT GATEWAY II server ensures the connection to the HEUFT TeleService for remote maintenance and the HEUFT PROFILER advanced the continuous recording, network-based transmission and long-term archiving of operating, production as well as batch data and detection images. The compact system for an empty vial inspection therefore fulfils the fundamental documentation and validation obligations in accordance with GMP and GAMP5 – and provides effective protection against contaminated vials and unnecessary vaccine waste.